Does Dna Replicate Again for Meiosis Ii
Jail cell Partitioning - Meiosis
From Embryology
Jump to:navigation, search
| Embryology - eighteen May 2022 |
|---|
| Google Translate - select your language from the listing shown below (this volition open a new external folio) |
| العربية | català | 中文 | 中國傳統的 | français | Deutsche | עִברִית | हिंदी | bahasa Indonesia | italiano | 日本語 | 한국어 | မြန်မာ | Pilipino | Polskie | português | ਪੰਜਾਬੀ ਦੇ | Română | русский | Español | Swahili | Svensk | ไทย | Türkçe | اردو | ייִדיש | Tiếng Việt These external translations are automated and may non exist accurate. (More than? About Translations) |
Introduction
Meiosis is the special blazon of recombinative and reductive cell sectionalisation occurring simply in the generation of the gametes or germ cells (oocyte and spermatozoa).
For recombination, meiosis requires that homologous chromosomes are properly paired and aligned past the induction of DNA double-strand breaks by the enzyme SPO11 during the prophase of the outset meiotic division.
Meiotic cell division also reduces (halves) the chromosomal content. The overall process of germ cell development is called "gametogenesis" and includes non just meiosis but also the cellular morphological changes, that occur differently in male person and female person gametes.
Some Contempo Findings
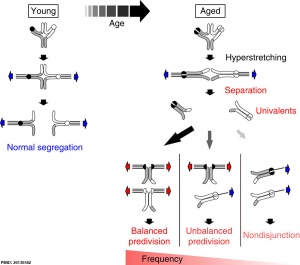
Bivalent separation into univalents precedes historic period-related meiosis I errors in oocytes[i]
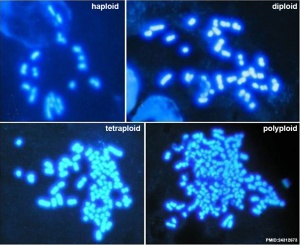
Karyotype of parthenogenetic blastocysts[two]
- Distinct prophase arrest mechanisms in human being male person meiosis [3] "To preclude chromosomal aberrations being transmitted to the offspring, strict meiotic checkpoints are in place to remove aberrant spermatocytes. Still, in about 1% of males these checkpoints cause complete meiotic arrest leading to azoospermia and subsequent infertility. Hither, nosotros unravel two clearly distinct meiotic arrest mechanisms that occur during prophase of homo male meiosis. Type I arrested spermatocytes display severe asynapsis of the homologous chromosomes, disturbed XY-trunk formation and increased expression of the Y chromosome-encoded factor c and seem to activate a Deoxyribonucleic acid damage pathway leading to induction of p63, peradventure causing spermatocyte apoptosis. Type II arrested spermatocytes display normal chromosome synapsis, normal XY-body morphology and meiotic crossover germination but have a lowered expression of several cell bike regulating genes and fail to silence the 10 chromosome-encoded gene ZFX."
- Review - Actin cytoskeleton dynamics in mammalian oocyte meiosis [four] "During mitosis, cells undergo symmetrical jail cell division, while oocyte meiotic maturation undergoes two consecutive, asymmetric divisions that generate a totipotent haploid oocyte and 2 small polar bodies not involved in DNA replication. This specialized division allows most maternal components be maintained in the oocytes for early embryo evolution. Nuclear positioning, germinal vesicle breakdown, spindle migration, spindle rotation, chromosome segregation, and polar body extrusion are the nigh critical cellular processes during oocyte meiosis I and Ii, and a growing number of studies primarily using the mouse oocyte model revealed that actin filaments were critical for these processes, especially for spindle migration. Several of import molecules take been reported to be involved in these processes. 1 family of molecules are the small GTPases, such as Rho GTPases, Ran GTPases, and Rab GTPases and another are the actin nucleators, such equally the formin family unit and the Arp2/3 complex. The nowadays review summarizes recent progress made regarding the roles of actin filaments in the asymmetric oocyte division." oocyte | polar body
- Inefficient Crossover Maturation Underlies Elevated Aneuploidy in Human Female Meiosis [five] "Meiosis is the cellular plan that underlies gamete germination. For this program, crossovers between homologous chromosomes play an essential mechanical role to ensure regular segregation. We present a detailed study of crossover germination in human male and female meiosis, enabled by modeling assay. Results suggest that recombination in the ii sexes proceeds analogously and efficiently through most stages. Even so, specifically in female person (but non male person), ∼25% of the intermediates that should mature into crossover products actually neglect to do then. Further, this "female person-specific crossover maturation inefficiency" is inferred to brand major contributions to the high level of chromosome mis-segregation and resultant aneuploidy that uniquely afflicts human female oocytes (eastward.g., giving Down syndrome). Additionally, crossover levels on different chromosomes in the same nucleus tend to co-vary, an effect attributable to global per-nucleus modulation of chromatin loop size. Maturation inefficiency could potentially reflect an evolutionary advantage of increased aneuploidy for human females."
- Contradistinct Crossover Distribution and Frequency in Spermatocytes of Infertile Men with Azoospermia [6] "Our study aims to narrate the crossover distribution in infertile men with non-obstructive (NOA) and obstructive azoospermia (OA) forth chromosomes 13, xviii and 21. Viii of the 16 NOA men and five of the 21 OA men in our study displayed reduced crossover frequency compared to command fertile men. Seven NOA men and nine OA men showed contradistinct crossover distributions on at least ane of the chromosome arms studied compared to controls. We establish that although both NOA and OA men displayed altered crossover distributions, NOA men may be at a higher risk of suffering both altered crossover frequencies and distributions compared to OA men."
- Review - The evolution of meiotic sex activity and its alternatives [7] "Meiosis is an ancestral, highly conserved process in eukaryotic life cycles, and for all eukaryotes the shared component of sexual reproduction. The benefits and functions of meiosis, all the same, are even so under discussion, especially considering the costs of meiotic sex."
|
|
Movies
A mouse oocyte undergoing meiosis spindle migration followed by start polar body extrusion and MII spindle positioning.[12]
The video shows that cytoplasmic streaming continues to the MII arrest stage to maintain the oocyte prepare of chromosomes/MII spindle in place close to the cortex. Frames are 11 min apart, and video length is 840 min. Bar, 20 µm. | |
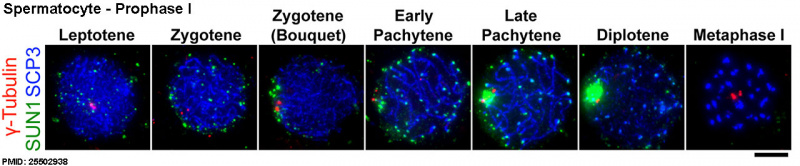
| A mouse spermatocyte undergoing meiosis prophase I. The chromosomal telomeres and synaptonemal complexes have been labelled to visualise chromosomal movement inside this single nucleus.[xiii] |
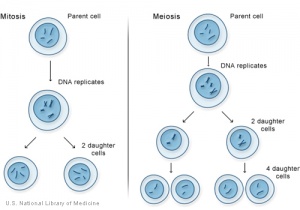
Comparison of Meiosis/Mitosis
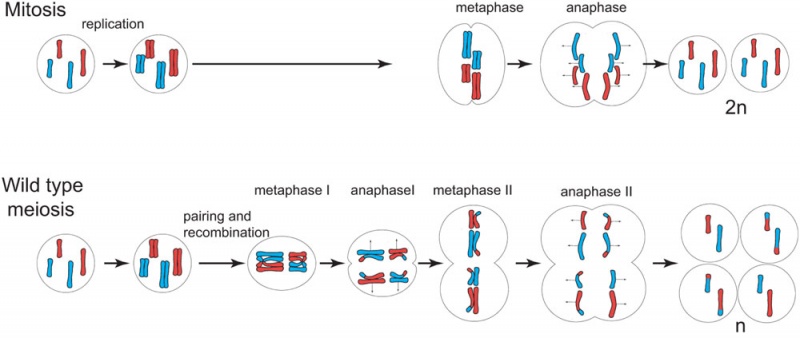
- After DNA replication ii nuclear (and jail cell) divisions required to produce haploid gametes
- Each diploid cell in meiosis produces 4 haploid cells (sperm) one haploid jail cell (egg)
- Each diploid cell mitosis produces 2 diploid cells
Meiosis Germ cell partition (haploid)
- Reductive division
- Generates haploid gametes (egg, sperm)
- Each genetically distinct from parent
- Genetic recombination (prophase ane)
- Exchanges portions of chromosomes maternal/paternal homologous pairs
- Independent assortment of paternal chromosomes (meiosis ane)
Homologous chromosomes pairing unique to meiosis
- Each chromosome duplicated and exists every bit fastened sister chromatids before pairing occurs
- Genetic Recombination shown by chromosomes part red and part black
- chromosome pairing in meiosis involves crossing-over between homologous chromosomes
Meiosis I and 2
- Meiosis I separates the pairs of homologous chromosomes, reduces the prison cell from diploid to haploid.
- Meiosis II separates each chromosome into two chromatids (chromosome behavior in meiosis II is like that of mitosis).
Links: Figure xiv.32. Comparison of meiosis and mitosis
Prophase I
prophase I
- The homologous chromosomes pair and commutation DNA to form recombinant chromosomes.
- Note - in oocyte development, from nascence until puberty oocytes are in "prophase I abort" at diplotene stage. This is important for sustaining the ovarian oocyte puddle and lutenizing hormone (LH) induces resumption of meiosis I.
 |
| |
Prophase I is further divided into five stages (phases):
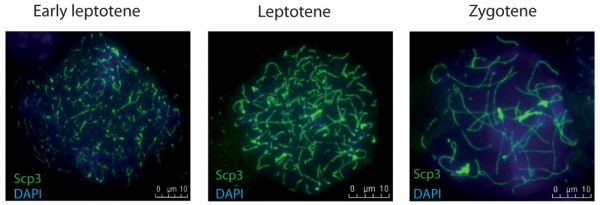
Leptotene
- leptotene phase, leptonema; Greek, leptotene = "thin threads"
- the duplicated paired chromosome homologs condense.
Zygotene
- zygotene stage, zygonema, Greek, zygotene = "paired threads"
- homologous chromosomes go closely associated (synapsis) to form pairs of chromosomes consisting of four chromatids (tetrads).
- the synaptonemal complex begins to course betwixt the two sets of sister chromatids in each bivalent (the duplicated chromosome paired with its homologous duplicated chromosome).
Pachytene
- pachytene phase, pachynema; Greek, pachytene = "thick threads"
- crossing over betwixt pairs of homologous chromosomes (meiotic recombination or synapsis) to form chiasmata (grade between 2 nonsister chromatids at points where they have crossed over)
- synaptonemal complex is complete and can be stable for some time.
- Autosomal non-sister chromatids of homologous chromosomes can at present extensively exchange segments in regions of homology.
- Only small regions of non-paired sexual activity chromosomes collaborate
- Mutations that compromise meiotic recombination in male spermatocytes upshot in arrest and apoptosis at this phase.
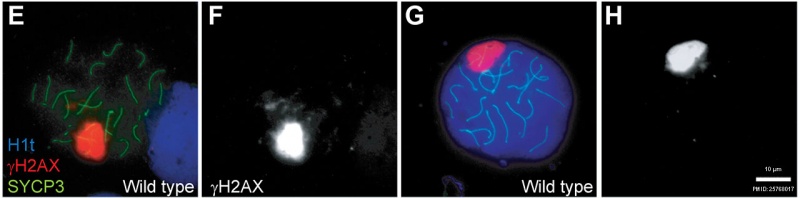
Mouse meiosis pachytene[15]
Diplotene
- diplotene stage, diplonema; Greek, diplonema = "ii threads"
- homologous chromosomes begin to separate but remain attached by chiasmata.
- synaptonemal complex degrades and the chromosomes separate from one another a small amount giving this appearance.
- It is possible that some chromosome uncoiling may also occur allowing some cistron transcription.
- In the developing human ovary, oocytes remain at the diplotene phase from fetal life through postnatal childhood, until puberty when the lutenizing hormone (LH) surges stimulate the resumption of meiosis.
Diakinesis
- diakinesis phase; Greek, diakinesis = "moving through"
- homologous chromosomes continue to separate, and chiasmata movement to the ends of the chromosomes.
- prophase I ends and chromosomes at present recondense, transcription stops and the transition to metaphase occurs.
Prometaphase I
- Spindle apparatus formed, and chromosomes attached to spindle fibres by kinetochores.
Metaphase I
- Homologous pairs of chromosomes (bivalents) arranged as a double row forth the metaphase plate. The arrangement of the paired chromosomes with respect to the poles of the spindle apparatus is random along the metaphase plate. (This is a source of genetic variation through random assortment, as the paternal and maternal chromosomes in a homologous pair are similar but not identical. The number of possible arrangements is 2n, where northward is the number of chromosomes in a haploid prepare. Human beings have 23 different chromosomes, and then the number of possible combinations is 223, which is over viii 1000000.)
Anaphase I
- The homologous chromosomes in each bivalent are separated and move to the opposite poles of the jail cell.
Telophase I
- The chromosomes become diffuse and the nuclear membrane reforms.
Cytokinesis I
- Cellular cytoplasmic partition to form two new cells, followed by Meiosis 2.
- Note - in oocyte meiosis, the extrusion of the starting time polar trunk (ane Pb) indicates completion of the offset meiotic division.
Prophase II
- Chromosomes brainstorm to condense, nuclear membrane breaks downwards and spindle forms.
Metaphase Two
- Spindle fibres attach to chromosomes, chromosomes align in prison cell middle.
Anaphase Ii
- Chromosomes separate and move to the reverse poles of the cell.
Telophase 2
- Chromosomes reach spindle pole ends and the nuclear membrane reforms.
Cytokinesis
Cellular cytoplasmic division to form new cells.
Meiosis Sexual activity Differences
Female person (oogenesis)
- Meiosis initiated in one case in a finite population of cells
- 1 gamete produced / meiosis
- Completion of meiosis delayed for months or years
- Meiosis arrested at 1st meiotic prophase and reinitiated in a smaller population of cells
- Differentiation of gamete occurs while diploid in offset meiotic prophase
- All chromosomes exhibit equivalent transcription and recombination during meiotic prophase
Male (spermatogenesis)
- Meiosis initiated continuously in a mitotically dividing stem cell population
- 4 gametes produced / meiosis
- Meiosis completed in days or weeks
- Meiosis and differentiation proceed continuously without cell cycle arrest
- Differentiation of gamete occurs while haploid after meiosis ends
Sex chromosomes excluded from recombination and transcription during commencement meiotic prophase
Female Gametogenesis
In females, the full number of eggs ever to be produced are present in the newborn female.
- All eggs are arrested at an early stage of the outset meiotic division as a primary oocyte (primordial follicle). Following purberty, during each menstrual cycle, pituitary gonadotrophin stimulates completion of meiosis i the twenty-four hour period before ovulation.
- In meiosis 1, a diploid jail cell becomes 2 haploid (23 chromosomes) daughter cells, each chromosome has two chromatids. One prison cell becomes the secondary oocyte the other jail cell forms the first polar torso.
- The secondary oocyte then commences meiosis ii which arrests at metaphase and will not go along without fertilization.
- At fertilization meiosis 2 completes, forming a second polar body. Note that the first polar body may too undergo this procedure forming a third polar body.
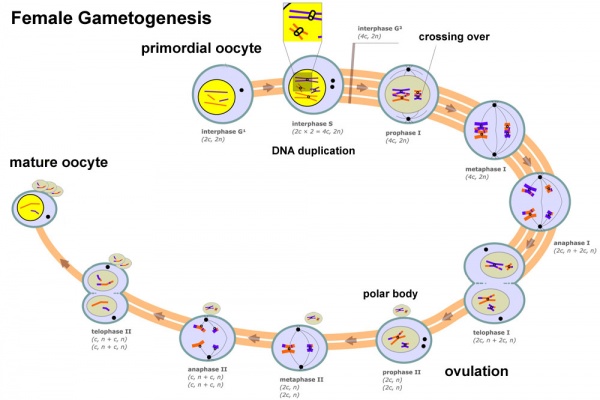
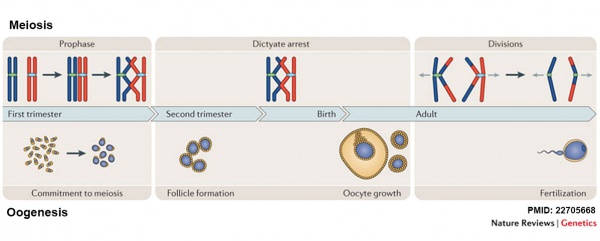 Meiosis and Oogenesis[17] | Meiosis - divided into iii temporally distinct phases.
|
| Oocyte Meiotic Spindles |
|---|
|
Polar Body
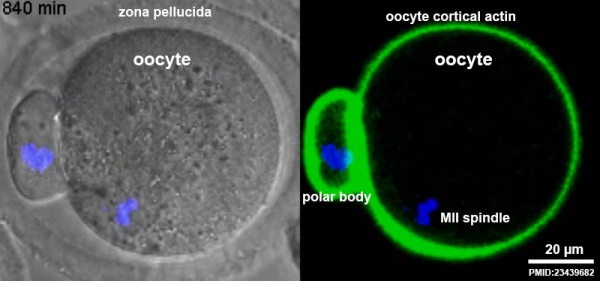
First Polar Body[12]
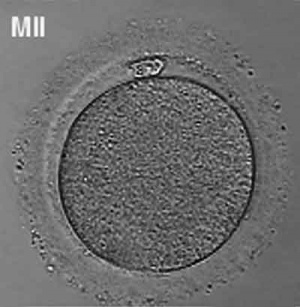
Human oocyte at metaphase II showing polar trunk at 12 o'clock position.
The breakdown of the germinal vesicle indicates a resumption of meiosis and the extrusion of the beginning polar body (i Pb) indicates completion of the first meiotic division in human oocytes. The polar body is a minor cytoplasmic exclusion body formed to enclose the backlog DNA formed during the oocyte (egg) meiosis and following sperm fertilization. At that place are 2-3 polar bodies derived from the oocyte present in the zygote, the number is dependent upon whether polar body 1 (the first polar trunk formed during meiosis 1) divides during meiosis 2. This exclusion body contains the backlog DNA from the reductive partitioning (the 2nd and third polar bodies are formed from meiosis 2 at fertilization). These polar bodies do not contribute to the future genetic complement of the zygote, embryo or fetus.
Recent inquiry in some species suggest that the infinite formed by the peripheral polar trunk (between the oocyte and the zona pellucia) can influence the site of spermatozoa fertilization.
Assisted reproductive techniques involving intracytoplasmic sperm injection (ICSI) have looked at the "quality" of the polar body and constitute that the morphology is related to mature oocyte viability and has the potential to predict oocyte fertilization rates and pregnancy accomplishment.[eighteen] [19]
- Polar Body Electron Micrographs
-
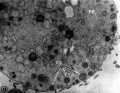
First Polar Torso cortical granules
-
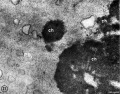
Commencement Polar Torso chromosomes
-
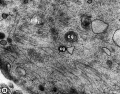
First Polar Body microtubules
-
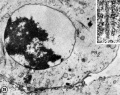
Second Polar Trunk chromatin
-

Second Polar Body cytoplasmic bridge
- Links: Category:Polar Torso
Female Abnormalities
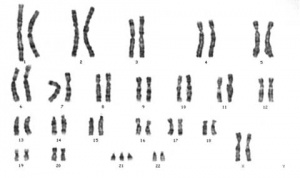
Trisomy 21 female karyotype
Meiotic non-disjunction resulting in aneuploidy, nigh are embryonic lethal and not seen. The potential for genetic abnormalities increment with maternal historic period.
- Autosomal chromosome aneuploidy
- Trisomy 21 - Down syndrome
- Trisomy 18 - Edwards syndrome
- Trisomy thirteen - Patau syndrome
- Sexual activity chromosome aneuploidy
- Monosomy Ten - Turner's Syndrome
- trisomy X - Triple-Ten syndrome
- 47 XXY - Klinefelter's Syndrome
Male Gametogenesis
In males, sperm continues to be generated throughout life from a stem cell population in the testis. Spermatozoa maturation involves ii processes meiosis and spermiogenesis 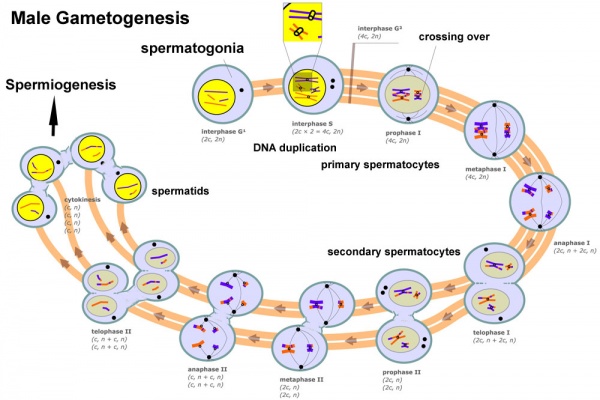
The higher up figure compares meiosis to the female (the polar bodies accept been removed and labelling updated).
-
Historic testis drawing
-
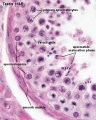
Adult Seminiferous tubule showing spermatozoa developmental stages
-
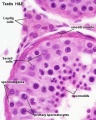
Seminiferous tubule cross-section and supporting cells
-
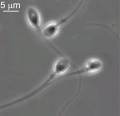
Human being spermatozoa
Human Spermatozoa Development
- Spermatogenesis procedure of spermatagonia mature into spermatazoa (sperm).
- Continuously throughout life occurs in the seminiferous tubules in the male gonad- testis (plural testes).
- At puberty spermatagonia activate and proliferate (mitosis).
- about 48 days from entering meiosis until morphologically mature spermatozoa
- about 64 days to complete spermatogenesis, depending reproduction time of spermatogonia
- follicle stimulating hormone (FSH) - stimulates the spermatogenic epithelium
- luteinizing-hormone (LH) - stimulates testosterone product past Leydig cells
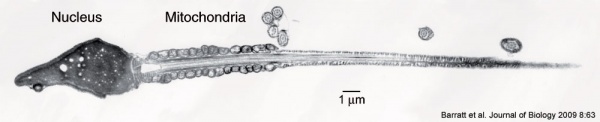
| | Mature human spermatozoa
|
- Links: Spermatozoa Development
Puberty
- In humans at puberty, hormonal and morphological changes occur inside the gonad and other systems (secondary sex characteristics).
- Within the testis the immature Sertoli cells cease to proliferate and differentiate.
- Spermatogonium proliferate and spermatogenesis begins.
- It takes near 70 days for cells to mature from the diploid spermatogonium to a primary spermatocyte.
- This maturation occurs in waves along the seminiferous tubules.
- Links: Puberty
Ejeculate
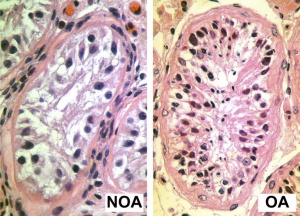
Azoospermia - Non-obstructive azoospermia (NOA) and Obstructive azoospermia (OA)
- release of spermatozoa and accessory gland secretions from the male person genital tract (iii.5 ml)
- 200-600 million sperm, by volume less than ten % spermatozoa
- Accessory Gland secretions - 60 % seminal vesicle, 30 % prostate and 10 % bulbourethral
Male person Abnormalities
- Oligospermia - (Low Sperm Count) less than 20 million sperm after 72 hour forbearance from sexual activity
- Azoospermia - (Absent Sperm) blockage of duct network
- Immotile Cilia Syndrome - lack of sperm motility
Meiosis in Other Species
- Ocean urchin - oocytes complete meiosis earlier being shed.
- Starfish - oocytes only complete meiosis upon hormonal stimulation.
Abnormalities
Meiotic Nondisjunction
- Occurs when homologues neglect to divide during meiotic sectionalization I or II
- For instance trisomy 21 (Downwardly Syndrome) caused past an extra copy of chromosome 21
- Links: trisomy 21 | Nondisjunction
Chromosomal Translocations
- Philadelphia chromosome
- Chronic myelogenous leukemia
- Piece of Chr9 exchanged with Chr22 Generates truncated abl
Overstimulates cell production
References
- ↑ ane.0 1.1 Sakakibara Y, Hashimoto S, Nakaoka Y, Kouznetsova A, Höög C & Kitajima TS. (2015). Bivalent separation into univalents precedes historic period-related meiosis I errors in oocytes. Nat Commun , 6, 7550. PMID: 26130582 DOI.
- ↑ 2.0 ii.ane Lin T, Diao YF, Kang JW, Lee JE, Kim DK & Jin DI. (2013). Chromosomes in the porcine showtime polar body possess competence of second meiotic segmentation within enucleated MII stage oocytes. PLoS One , 8, e82766. PMID: 24312673 DOI.
- ↑ Jan SZ, Jongejan A, Korver CM, van Daalen SKM, van Pelt AMM, Repping S & Hamer G. (2018). Distinct prophase abort mechanisms in human male meiosis. Development , 145, . PMID: 29540502 DOI.
- ↑ Duan Ten & Lord's day SC. (2018). Actin cytoskeleton dynamics in mammalian oocyte meiosis. Biol. Reprod. , , . PMID: 30010726 DOI.
- ↑ Wang S, Hassold T, Hunt P, White MA, Zickler D, Kleckner N & Zhang Fifty. (2017). Inefficient Crossover Maturation Underlies Elevated Aneuploidy in Human Female Meiosis. Cell , 168, 977-989.e17. PMID: 28262352 DOI.
- ↑ Ren H, Ferguson Yard, Kirkpatrick G, Vinning T, Grub Five & Ma South. (2016). Contradistinct Crossover Distribution and Frequency in Spermatocytes of Infertile Men with Azoospermia. PLoS One , 11, e0156817. PMID: 27273078 DOI.
- ↑ Mirzaghaderi G & Hörandl E. (2016). The development of meiotic sexual activity and its alternatives. Proc. Biol. Sci. , 283, . PMID: 27605505 DOI.
- ↑ Bury L, Coelho PA & Glover DM. (2016). From Meiosis to Mitosis: The Astonishing Flexibility of Cell Partition Mechanisms in Early Mammalian Development. Curr. Superlative. Dev. Biol. , 120, 125-71. PMID: 27475851 DOI.
- ↑ Royo H, Seitz H, ElInati Eastward, Peters AH, Stadler MB & Turner JM. (2015). Silencing of X-Linked MicroRNAs by Meiotic Sex Chromosome Inactivation. PLoS Genet. , 11, e1005461. PMID: 26509798 DOI.
- ↑ Yun Y, Lane SI & Jones KT. (2014). Premature dyad separation in meiosis II is the major segregation mistake with maternal age in mouse oocytes. Evolution , 141, 199-208. PMID: 24346700 DOI.
- ↑ Zhai R, Yuan YF, Zhao Y, Liu XM, Zhen YH, Yang FF, Wang L, Huang CZ, Cao J & Huo LJ. (2013). Bora regulates meiotic spindle assembly and cell wheel during mouse oocyte meiosis. Mol. Reprod. Dev. , 80, 474-87. PMID: 23610072 DOI.
- ↑ 12.0 12.1 Yi Thou, Rubinstein B, Unruh JR, Guo F, Slaughter BD & Li R. (2013). Sequential actin-based pushing forces bulldoze meiosis I chromosome migration and symmetry breaking in oocytes. J. Cell Biol. , 200, 567-76. PMID: 23439682 DOI.
- ↑ Shibuya H, Morimoto A & Watanabe Y. (2014). The dissection of meiotic chromosome movement in mice using an in vivo electroporation technique. PLoS Genet. , 10, e1004821. PMID: 25502938 DOI.
- ↑ Boku T, Nakane Y, Komada H, Nakagawa M, Hirozane Due north, Hioki Yard & Yamamoto M. (1991). [Prognostic factors of "pm" gastric cancer]. Nihon Geka Gakkai Zasshi , 92, 17-23. PMID: 2014022
- ↑ Pacheco Southward, Marcet-Ortega Thousand, Lange J, Jasin G, Keeney Due south & Roig I. (2015). The ATM signaling cascade promotes recombination-dependent pachytene abort in mouse spermatocytes. PLoS Genet. , xi, e1005017. PMID: 25768017 DOI.
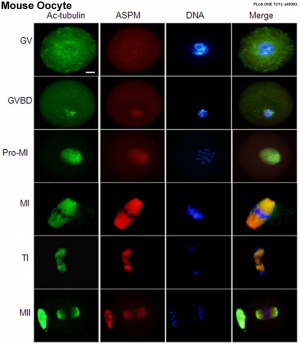
- ↑ Xu XL, Ma West, Zhu YB, Wang C, Wang BY, An N, An 50, Liu Y, Wu ZH & Tian JH. (2012). The microtubule-associated protein ASPM regulates spindle assembly and meiotic progression in mouse oocytes. PLoS I , 7, e49303. PMID: 23152892 DOI.
- ↑ Nagaoka SI, Hassold TJ & Hunt PA. (2012). Human aneuploidy: mechanisms and new insights into an historic period-old trouble. Nat. Rev. Genet. , 13, 493-504. PMID: 22705668 DOI.
- ↑ Ebner T, Yaman C, Moser Grand, Sommergruber Yard, Feichtinger O & Tews G. (2000). Prognostic value of first polar body morphology on fertilization charge per unit and embryo quality in intracytoplasmic sperm injection. Hum. Reprod. , 15, 427-30. PMID: 10655316
- ↑ Younis JS, Radin O, Izhaki I & Ben-Ami M. (2009). Does offset polar torso morphology predict oocyte performance during ICSI handling?. J. Aid. Reprod. Genet. , 26, 561-seven. PMID: 19960239 DOI.
Textbooks
- MBoC - Sperm | MBoC - Highly simplified drawing of a cross-section of a seminiferous tubule in a mammalian testis | MBoC - Cytoplasmic bridges in developing sperm cells and their precursors
Podcasts
- Biosights 18 March 2013 - Breaking egg symmetry
Reviews
Greaney J, Wei Z & Homer H. (2017). Regulation of chromosome segregation in oocytes and the cellular basis for female person meiotic errors. Hum. Reprod. Update , , . PMID: 29244163 DOI.
Brar GA & Amon A. (2008). Emerging roles for centromeres in meiosis I chromosome segregation. Nat. Rev. Genet. , 9, 899-910. PMID: 18981989 DOI.
Articles
Zelazowski MJ, Sandoval M, Paniker Fifty, Hamilton HM, Han J, Gribbell MA, Kang R & Cole F. (2017). Age-Dependent Alterations in Meiotic Recombination Crusade Chromosome Segregation Errors in Spermatocytes. Cell , 171, 601-614.e13. PMID: 28942922 DOI.
Ren H, Ferguson One thousand, Kirkpatrick M, Vinning T, Chow V & Ma Southward. (2016). Contradistinct Crossover Distribution and Frequency in Spermatocytes of Infertile Men with Azoospermia. PLoS 1 , 11, e0156817. PMID: 27273078 DOI.
Wang Southward, Kleckner Northward & Zhang Fifty. (2017). Crossover maturation inefficiency and aneuploidy in human female meiosis. Cell Cycle , sixteen, 1017-1019. PMID: 28471715 DOI.
Search Pubmed
Search Pubmed: meiosis
NCBI - Policies and Guidelines | PubMed | Assistance:Reference Tutorial
Additional Images
-
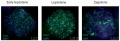
Meiotic prophase I stages
-
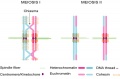
Chromosome connections in meiosis
-

Kinetochore attachment to the spindle and chromosome cohesion in mitosis and meiosis
-
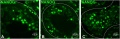
Human testis NANOG expression
-
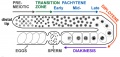
Adult worm hermaphrodite gonad arm
-
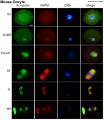
Mouse oocyte microtubule-associated protein
Terms
| Cell Sectionalization Terms (aggrandize to view) | ||
|---|---|---|
meiosis | mitosis
| ||
|
External Links
External Links Detect - The dynamic nature of the net may mean that some of these listed links may no longer function. If the link no longer works search the web with the link text or proper noun. Links to any external commercial sites are provided for information purposes only and should never be considered an endorsement. UNSW Embryology is provided equally an educational resource with no clinical information or commercial affiliation.
Glossary Links
- Glossary: A | B | C | D | East | F | G | H | I | J | K | Fifty | Thou | North | O | P | Q | R | Southward | T | U | V | West | Ten | Y | Z | Numbers | Symbols | Term Link
Cite this page: Hill, G.A. (2022, May 18) Embryology Cell Division - Meiosis. Retrieved from https://embryology.med.unsw.edu.au/embryology/index.php/Cell_Division_-_Meiosis
-
- What Links Here?
- © Dr Mark Colina 2022, UNSW Embryology ISBN: 978 0 7334 2609 four - UNSW CRICOS Provider Code No. 00098G
Source: https://embryology.med.unsw.edu.au/embryology/index.php/Cell_Division_-_Meiosis

0 Response to "Does Dna Replicate Again for Meiosis Ii"
Postar um comentário