Unit 2 Matter and Energy Lesson 6 Periodic Table Families Answer Key
Chapter 2. Atoms, Molecules, and Ions
2.5 The Periodic Tabular array
Learning Objectives
By the end of this section, you will be able to:
- Land the periodic police force and explicate the organization of elements in the periodic table
- Predict the full general backdrop of elements based on their location within the periodic table
- Identify metals, nonmetals, and metalloids by their properties and/or location on the periodic table
Equally early chemists worked to purify ores and discovered more elements, they realized that various elements could exist grouped together by their similar chemical behaviors. One such grouping includes lithium (Li), sodium (Na), and potassium (G): These elements all are shiny, carry heat and electricity well, and have similar chemic backdrop. A 2d group includes calcium (Ca), strontium (Sr), and barium (Ba), which too are shiny, skillful conductors of heat and electricity, and have chemic backdrop in common. However, the specific properties of these two groupings are notably different from each other. For example: Li, Na, and Grand are much more reactive than are Ca, Sr, and Ba; Li, Na, and K form compounds with oxygen in a ratio of 2 of their atoms to ane oxygen atom, whereas Ca, Sr, and Ba form compounds with one of their atoms to one oxygen cantlet. Fluorine (F), chlorine (Cl), bromine (Br), and iodine (I) also exhibit similar properties to each other, but these properties are drastically different from those of whatsoever of the elements above.
Dimitri Mendeleev in Russia (1869) and Lothar Meyer in Deutschland (1870) independently recognized that there was a periodic relationship among the backdrop of the elements known at that time. Both published tables with the elements arranged according to increasing diminutive mass. Just Mendeleev went i pace further than Meyer: He used his table to predict the existence of elements that would have the properties similar to aluminum and silicon, but were yet unknown. The discoveries of gallium (1875) and germanium (1886) provided nifty support for Mendeleev's work. Although Mendeleev and Meyer had a long dispute over priority, Mendeleev'southward contributions to the development of the periodic table are now more than widely recognized (Figure 1).
Past the twentieth century, information technology became apparent that the periodic relationship involved atomic numbers rather than atomic masses. The modernistic statement of this relationship, the periodic law, is as follows: the properties of the elements are periodic functions of their atomic numbers. A modern periodic table arranges the elements in increasing order of their diminutive numbers and groups atoms with similar properties in the same vertical column (Figure 2). Each box represents an element and contains its atomic number, symbol, boilerplate atomic mass, and (sometimes) proper name. The elements are arranged in vii horizontal rows, called periods or serial, and 18 vertical columns, called groups. Groups are labeled at the top of each cavalcade. In the U.s.a., the labels traditionally were numerals with capital letters. Even so, IUPAC recommends that the numbers 1 through 18 be used, and these labels are more common. For the table to fit on a single folio, parts of two of the rows, a full of xiv columns, are usually written below the main body of the table.
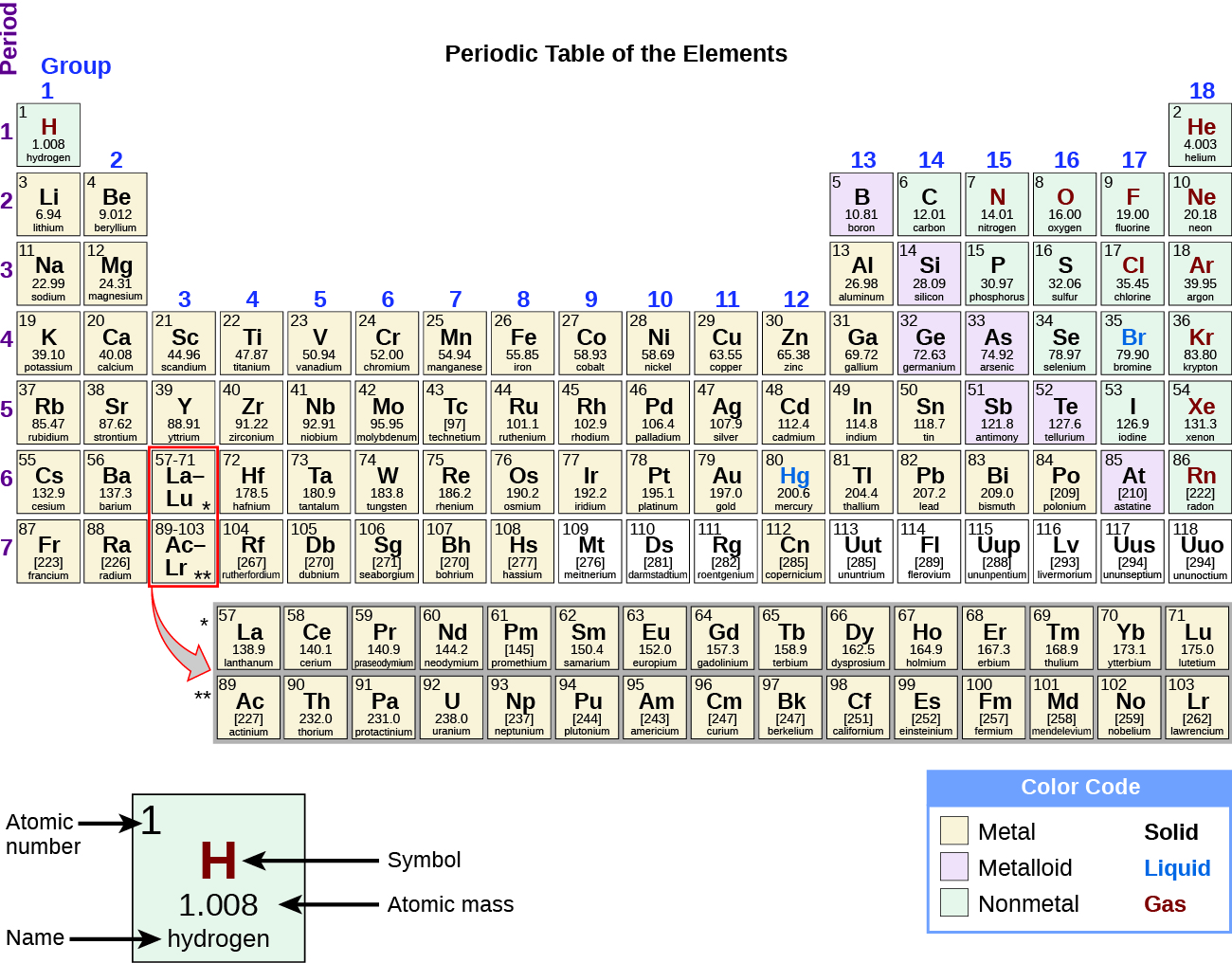
Many elements differ dramatically in their chemical and physical properties, but some elements are like in their behaviors. For example, many elements appear shiny, are malleable (able to be deformed without breaking) and ductile (can exist drawn into wires), and conduct heat and electricity well. Other elements are not shiny, malleable, or ductile, and are poor conductors of heat and electricity. We can sort the elements into large classes with common properties: metals (elements that are shiny, malleable, skillful conductors of oestrus and electricity—shaded yellowish); nonmetals (elements that appear dull, poor conductors of estrus and electricity—shaded green); and metalloids (elements that conduct heat and electricity moderately well, and possess some properties of metals and some properties of nonmetals—shaded royal).
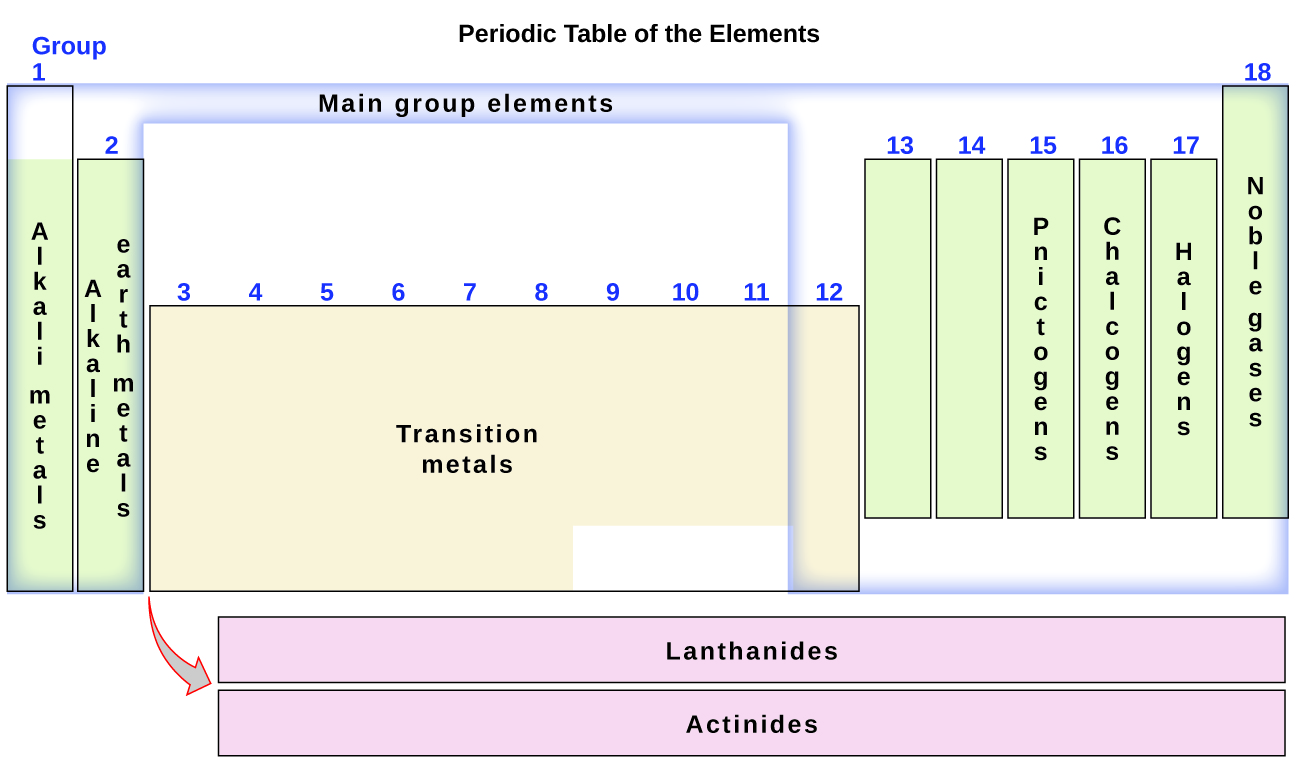
The elements tin also be classified into the main-group elements (or representative elements) in the columns labeled i, two, and 13–xviii; the transition metals in the columns labeled 3–12; and inner transition metals in the 2 rows at the bottom of the table (the summit-row elements are called lanthanides and the lesser-row elements are actinides; Figure 3). The elements can be subdivided further by more specific properties, such equally the limerick of the compounds they form. For instance, the elements in group 1 (the kickoff column) course compounds that consist of 1 atom of the element and 1 atom of hydrogen. These elements (except hydrogen) are known as alkali metals, and they all accept similar chemic properties. The elements in group 2 (the 2d column) form compounds consisting of one atom of the element and 2 atoms of hydrogen: These are chosen alkali metal earth metals, with similar properties amid members of that group. Other groups with specific names are the pnictogens (group 15), chalcogens (group 16), halogens (group 17), and the noble gases (group 18, likewise known every bit inert gases). The groups can as well exist referred to by the first element of the group: For example, the chalcogens can be chosen the oxygen group or oxygen family unit. Hydrogen is a unique, nonmetallic chemical element with properties similar to both grouping 1A and group 7A elements. For that reason, hydrogen may be shown at the top of both groups, or past itself.

Click on this link for an interactive periodic tabular array, which you tin can use to explore the backdrop of the elements (includes podcasts and videos of each chemical element). You may also want to attempt this 1 that shows photos of all the elements.
Instance 1
Naming Groups of Elements
Atoms of each of the following elements are essential for life. Give the group proper name for the post-obit elements:
(a) chlorine
(b) calcium
(c) sodium
(d) sulfur
Solution
The family unit names are as follows:
(a) halogen
(b) element of group i earth metal
(c) alkali metal
(d) chalcogen
Cheque Your Learning
Give the group name for each of the following elements:
(a) krypton
(b) selenium
(c) barium
(d) lithium
Answer:
(a) noble gas; (b) chalcogen; (c) alkaline earth metal; (d) alkali metal
In studying the periodic table, you might have noticed something nearly the atomic masses of some of the elements. Chemical element 43 (technetium), element 61 (promethium), and almost of the elements with atomic number 84 (polonium) and higher have their atomic mass given in square brackets. This is done for elements that consist entirely of unstable, radioactive isotopes (you volition acquire more than about radioactivity in the nuclear chemical science chapter). An average atomic weight cannot be determined for these elements because their radioisotopes may vary significantly in relative abundance, depending on the source, or may not even exist in nature. The number in foursquare brackets is the atomic mass number (and estimate atomic mass) of the most stable isotope of that chemical element.
Cardinal Concepts and Summary
The discovery of the periodic recurrence of like properties among the elements led to the formulation of the periodic tabular array, in which the elements are bundled in order of increasing atomic number in rows known as periods and columns known as groups. Elements in the aforementioned group of the periodic table have similar chemic backdrop. Elements tin can be classified every bit metals, metalloids, and nonmetals, or equally a master-grouping elements, transition metals, and inner transition metals. Groups are numbered i–18 from left to correct. The elements in group 1 are known as the alkali metals; those in grouping 2 are the alkaline earth metals; those in 15 are the pnictogens; those in 16 are the chalcogens; those in 17 are the halogens; and those in 18 are the noble gases.
Chemical science End of Chapter Exercises
- Using the periodic table, classify each of the following elements as a metal or a nonmetal, and then further classify each as a main-group (representative) element, transition metal, or inner transition element:
(a) uranium
(b) bromine
(c) strontium
(d) neon
(e) gold
(f) americium
(k) rhodium
(h) sulfur
(i) carbon
(j) potassium
- Using the periodic table, classify each of the following elements as a metal or a nonmetal, and then farther classify each as a principal-group (representative) element, transition metal, or inner transition metal:
(a) cobalt
(b) europium
(c) iodine
(d) indium
(east) lithium
(f) oxygen
(h) cadmium
(i) terbium
(j) rhenium
- Using the periodic table, identify the lightest member of each of the following groups:
(a) noble gases
(b) alkaline earth metals
(c) alkali metals
(d) chalcogens
- Using the periodic table, identify the heaviest member of each of the post-obit groups:
(a) alkali metals
(b) chalcogens
(c) noble gases
(d) alkaline globe metals
- Utilize the periodic table to requite the name and symbol for each of the following elements:
(a) the noble gas in the same period as germanium
(b) the alkaline earth metal in the aforementioned period as selenium
(c) the halogen in the same period as lithium
(d) the chalcogen in the same period every bit cadmium
- Utilise the periodic table to give the name and symbol for each of the following elements:>
(a) the element of group vii in the aforementioned menstruum every bit the alkali metal with xi protons
(b) the alkaline earth metallic in the same period with the neutral element of group 0 with 18 electrons
(c) the noble gas in the aforementioned row as an isotope with 30 neutrons and 25 protons
(d) the noble gas in the same period as gilded
- Write a symbol for each of the post-obit neutral isotopes. Include the diminutive number and mass number for each.
(a) the alkali metallic with 11 protons and a mass number of 23
(b) the element of group 0 element with 75 neutrons in its nucleus and 54 electrons in the neutral atom
(c) the isotope with 33 protons and xl neutrons in its nucleus
(d) the alkaline globe metallic with 88 electrons and 138 neutrons
- Write a symbol for each of the following neutral isotopes. Include the atomic number and mass number for each.
(a) the chalcogen with a mass number of 125
(b) the halogen whose longest-lived isotope is radioactive
(c) the noble gas, used in lighting, with x electrons and 10 neutrons
(d) the lightest alkali metal with 3 neutrons
Glossary
- actinide
- inner transition metal in the bottom of the bottom ii rows of the periodic table
- brine metallic
- element in group 1
- element of group ii
- element in group 2
- chalcogen
- element in group xvi
- grouping
- vertical cavalcade of the periodic table
- halogen
- chemical element in group 17
- inert gas
- (also, noble gas) element in group 18
- inner transition metallic
- (too, lanthanide or actinide) element in the bottom two rows; if in the first row, also called lanthanide, or if in the second row, also called actinide
- lanthanide
- inner transition metal in the top of the bottom 2 rows of the periodic table
- chief-group element
- (as well, representative chemical element) element in columns 1, two, and 12–18
- metal
- element that is shiny, malleable, good conductor of heat and electricity
- metalloid
- element that conducts rut and electricity moderately well, and possesses some properties of metals and some properties of nonmetals
- noble gas
- (also, inert gas) element in group 18
- nonmetal
- element that appears tedious, poor conductor of heat and electricity
- period
- (too, series) horizontal row of the periodic table
- periodic constabulary
- properties of the elements are periodic function of their atomic numbers.
- periodic table
- table of the elements that places elements with like chemical backdrop close together
- pnictogen
- chemical element in grouping 15
- representative chemical element
- (also, main-group chemical element) element in columns 1, 2, and 12–18
- series
- (as well, period) horizontal row of the menstruum table
- transition metal
- element in columns three–11
Solutions
Answers to Chemistry Stop of Chapter Exercises
1. (a) metal, inner transition metal; (b) nonmetal, representative element; (c) metal, representative element; (d) nonmetal, representative element; (e) metal, transition element; (f) metal, inner transition metal; (m) metal, transition metallic; (h) nonmetal, representative element; (i) nonmetal, representative chemical element; (j) metal, representative element
three. (a) He; (b) Exist; (c) Li; (d) O
5. (a) krypton, Kr; (b) calcium, Ca; (c) fluorine, F; (d) tellurium, Te
7. (a) [latex]_{11}^{23}\text{Na}[/latex]; (b) [latex]_{54}^{129}\text{Xe}[/latex]; (c) [latex]_{33}^{73}\text{As}[/latex] ; (d) [latex]_{88}^{226}\text{Ra}[/latex];
Source: https://opentextbc.ca/chemistry/chapter/2-5-the-periodic-table/
0 Response to "Unit 2 Matter and Energy Lesson 6 Periodic Table Families Answer Key"
Postar um comentário